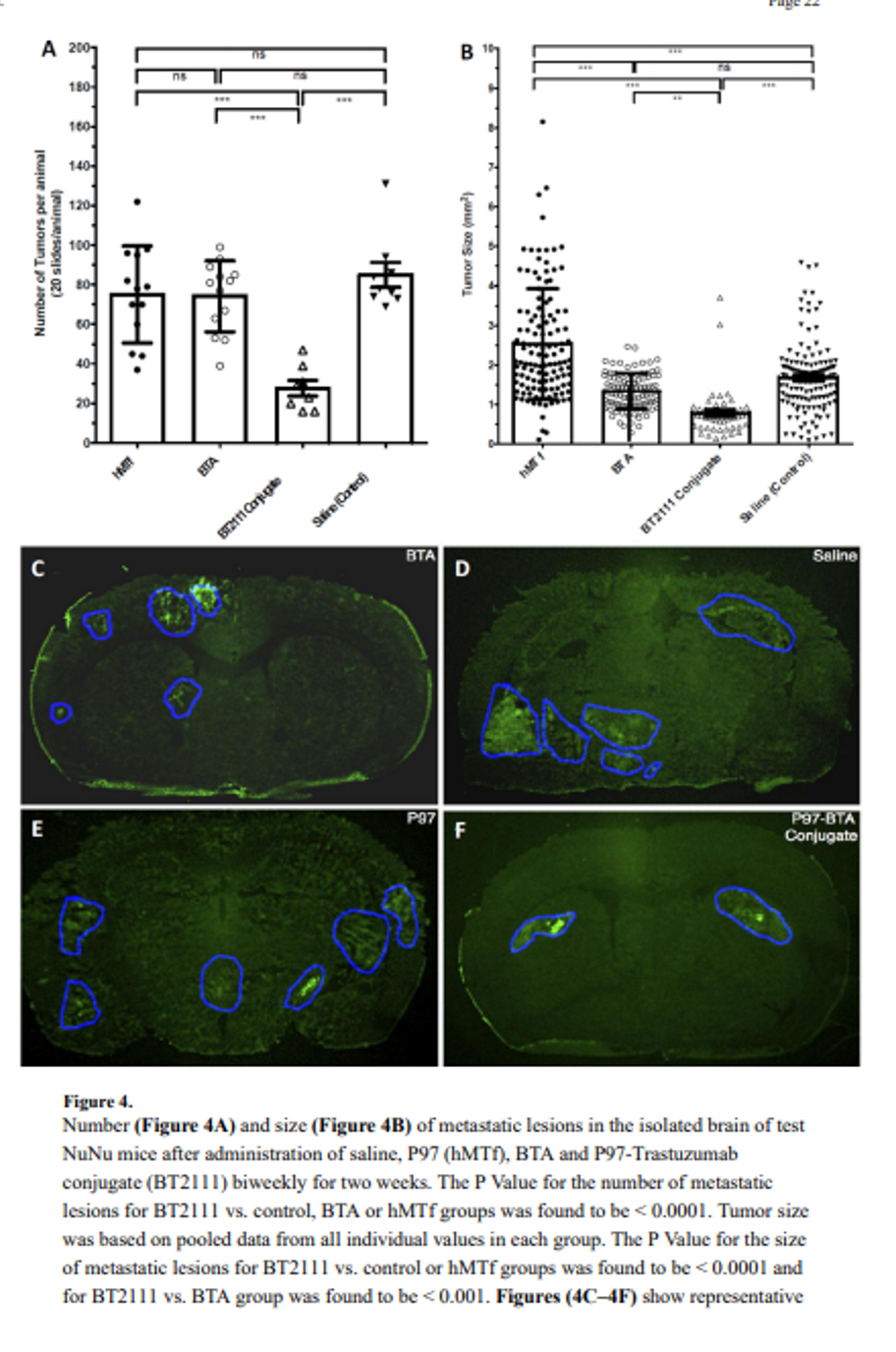
xB3 platform: Enabling Targeted CNS Delivery
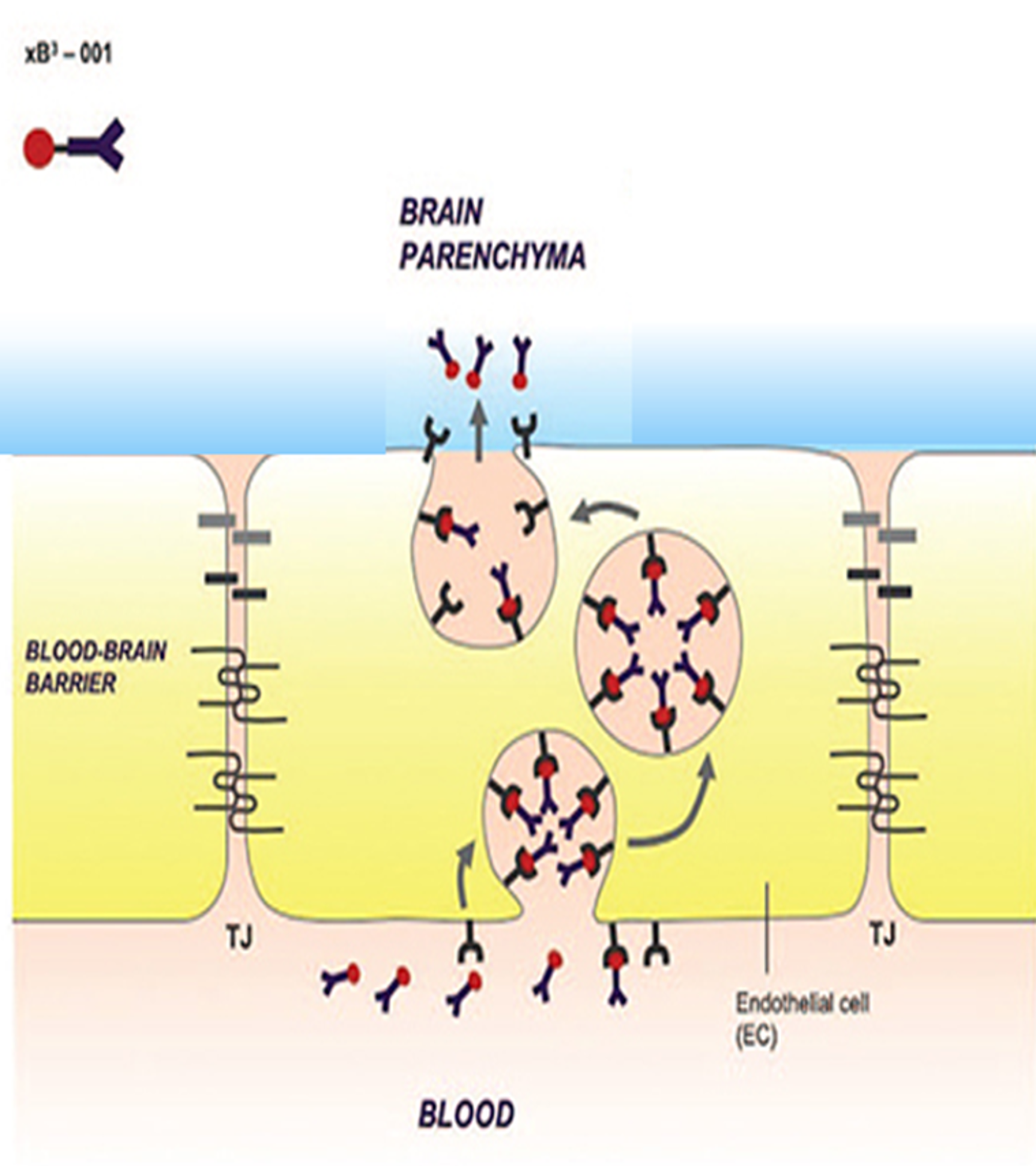
The xB3 platform is a proprietary technology designed to overcome one of the most significant barriers in neurology: the blood-brain barrier (BBB).
How xB3 Works
Derived from melanotransferrin, xB3 utilizes the low-density lipoprotein-related protein 1 (LRP1) as a receptor for its action. The LRP1 receptor is specifically expressed on the endothelium of cerebral vessels (Strickland & Muratoglu, Arteriosl Thromb Vasc Biol 2016). xB3 carries payloads across the BBB via a process of endocytosis and transcytosis.
Payload Flexibility
This transport is applicable to a variety of active molecules including antibodies, enzymes and oligonucleotides, with a potential for repurposing them in a tailored manner for CNS delivery drugs with proven pharmacological efficacy.
The xB3 technology is exclusively licensed from Bioasis Technology Inc, Canada/US, for use in neuroinflammatory conditions.