EGF plays a direct role in myelin synthesis
EGF is a natural peptide that plays a crucial role in tissue development and repair, including the central nervous system (CNS).
How EGF drives neuroregeneration
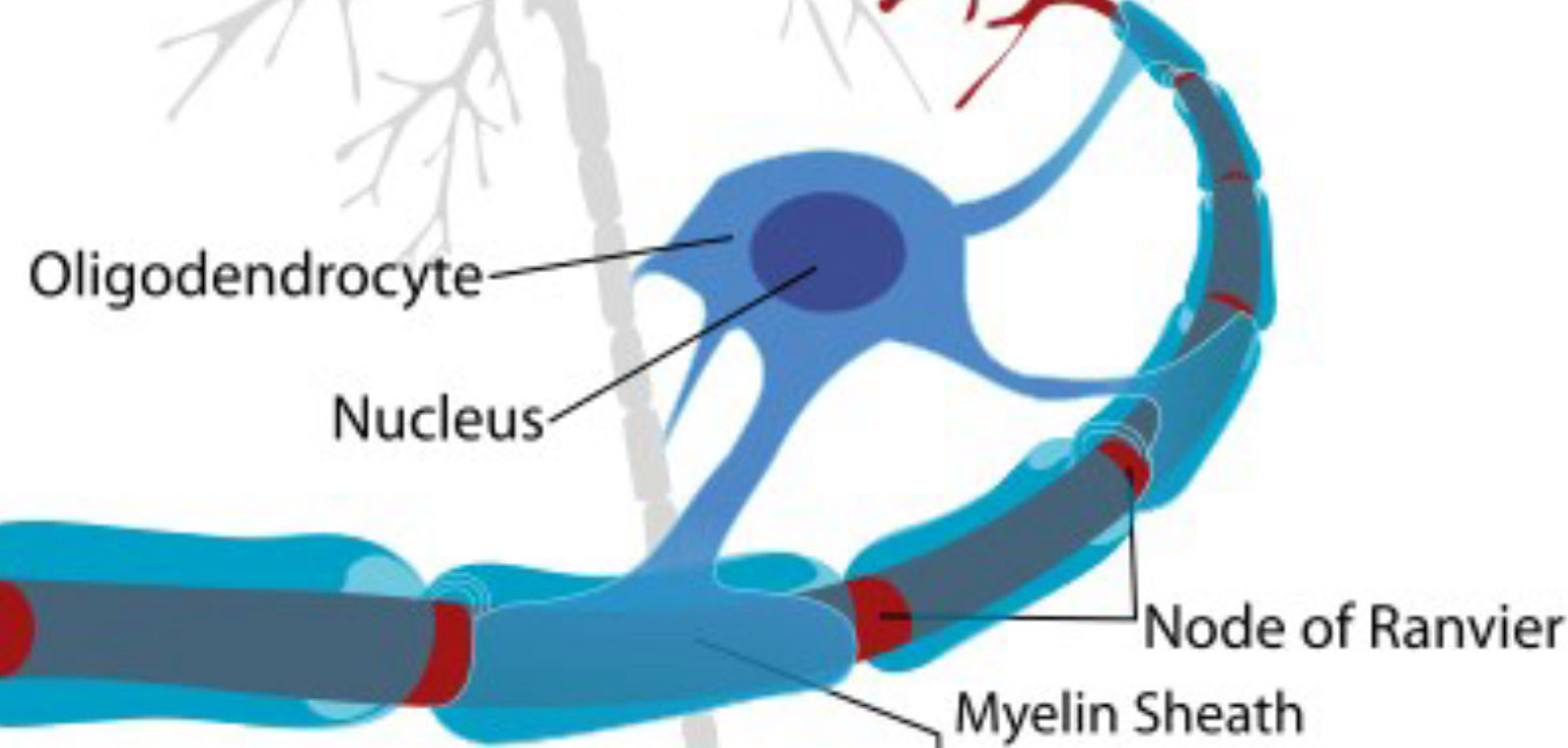
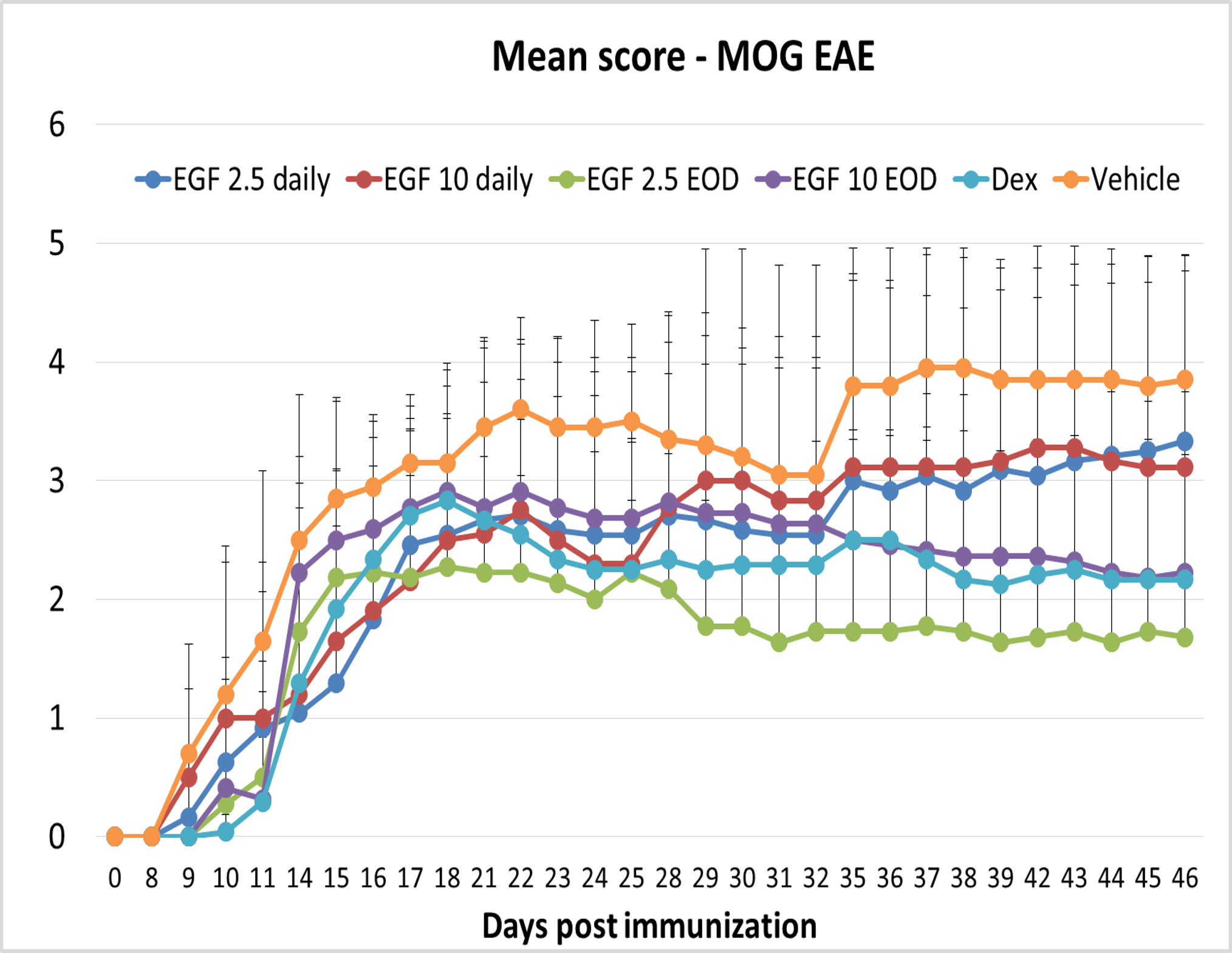
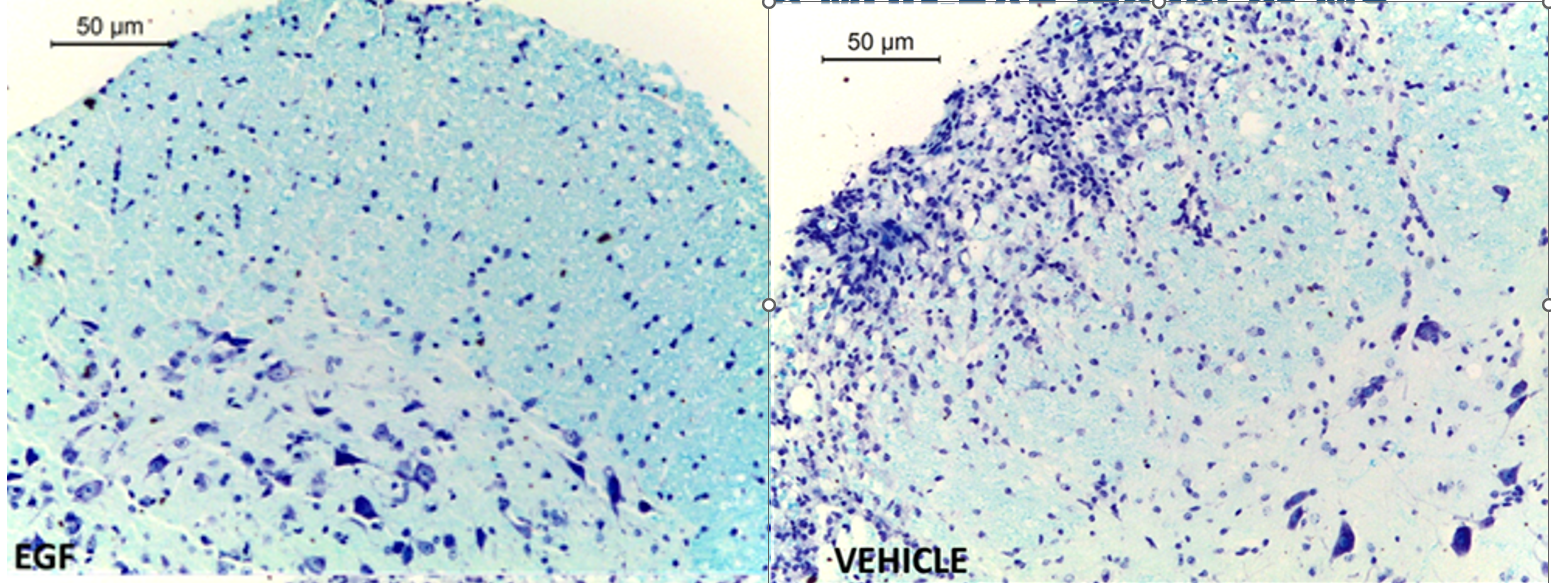
- EGF stimulates oligodendrocyte differentiation and maturation, a key step necessary for remyelination, as evident after CNS injury (Scalabrino, Front Neurol 2021). The remyelination effect of EGF has been shown by intraperitoneal administration of EGF in Multiple Sclerosis animal models.
- Its mode of action relies on stimulating oligodendrocyte and Schwann cell differentiation and maturation which represent key steps necessary for remyelination. This is seen after CNS injury models. Also, the EGF gene is strongly activated in the repair phase following Theiler’s virus-induced demyelination in mice and the EGF mRNA synthesis is significantly increased in the corpus callosum during remyelination after cuprizone-induced demyelination.
- Among its effects, EGF is the effector of the myelinotropic action of cobalamin (Cbl) and it positively regulates the myelinotropic PrPC protein synthesis which is essential for myelin maintenance.